reprinted with permission from Nature magazine
A
Structure for Deoxyribose Nucleic Acid
J.
D. Watson and F. H. C. Crick
(1)
April 25, 1953
(2),
Nature
(3)
,
171, 737-738
We wish to suggest a structure for the salt of deoxyribose
nucleic acid (D.N.A.). This structure has novel features which are of
considerable biological interest.
A structure for nucleic
acid has already been proposed by
Pauling (4)
and Corey
1
.
They kindly made their manuscript available to us in advance of publication.
Their model consists of three intertwined chains, with the phosphates
near the fibre axis, and the bases on the outside. In our opinion, this
structure is unsatisfactory for two reasons:
(1) We believe that
the material which gives the X-ray diagrams is the salt, not the free
acid. Without the acidic hydrogen atoms it is not clear what forces would
hold the structure together, especially as the negatively charged phosphates
near the axis will repel each other.
(2) Some of the van
der Waals distances appear to be too small.
Another three-chain
structure has also been suggested by Fraser (in the press). In his model
the phosphates are on the outside and the bases on the inside, linked
together by hydrogen bonds. This structure as described is rather ill-defined,
and for this reason we shall not comment on it.
We
wish to put forward a
radically different structure
for the salt of deoxyribose nucleic acid (5)
.
This structure has two helical chains each coiled round the same axis
(see diagram). We have made the usual chemical assumptions, namely, that
each chain consists of phosphate diester groups joining beta-D-deoxyribofuranose
residues with 3',5' linkages. The two chains (but not their bases) are
related by a dyad perpendicular to the fibre axis. Both chains follow
right-handed helices, but owing to the dyad the sequences of the atoms
in
the two chains run in opposite directions
(6)
. Each chain loosely resembles
Furberg's
2
model No. 1 (7)
; that is, the bases are on the inside of the
helix and the phosphates on the outside. The configuration of the sugar
and the atoms near it is close to Furberg's "standard configuration,"
the sugar being roughly perpendicular to the attached base. There is a
residue on each every 3.4 A. in the
z
-direction. We have assumed
an angle of 36° between adjacent residues in the same chain, so that
the structure repeats after 10 residues on each chain, that is, after
34 A. The distance of a phosphorus atom from the fibre axis is 10 A. As
the phosphates are on the outside, cations have easy access to them.
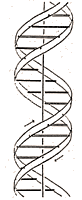
|
Figure
1
This figure is purely diagrammatic (8) . The two ribbons symbolize the two phophate-sugar chains, and the horizonal rods the pairs of bases holding the chains together. The vertical line marks the fibre axis. |
The structure is an open one,
and its water content is rather high. At lower water contents we would
expect the bases to tilt so that the structure could become more compact.
The novel feature of the structure is the manner in which the two chains
are held together by the purine and pyrimidine bases. The planes of the
bases are perpendicular to the fibre axis. They are joined together in
pairs, a single base from one chain being hydroden-bonded to a single
base from the other chain, so that the two lie side by side with identical
z
-coordinates. One of the pair must be a purine and the other
a pyrimidine for bonding to occur. The hydrogen bonds are made as follows:
purine position 1 to pyrimidine position 1; purine position 6 to pyrimidine
position 6.
If it is assumed that the bases only occur in the structure in the most plausible tautomeric forms (that is, with the keto rather than the enol configurations) it is found that only specific pairs of bases can bond together. These pairs are: adenine (purine) with thymine (pyrimidine), and guanine (purine) with cytosine (pyrimidine) (9) .
In other words, if an adenine
forms one member of a pair, on either chain, then on these assumptions
the other member must be thymine; similarly for guanine and cytosine.
The sequence of bases on a single chain does not appear to be restricted
in any way. However, if only specific pairs of bases can be formed, it
follows that if the sequence of bases on one chain is given, then the
sequence on the other chain is automatically determined.
It has been found experimentally
3,4
that the ratio of the amounts of adenine to thymine, and the ratio of
guanine to cytosine, are always very close to unity for deoxyribose nucleic
acid.
It is probably impossible to build this structure with a ribose sugar
in place of the deoxyribose, as the extra oxygen atom would make too close
a van der Waals contact.
The previously published X-ray data
5,6
on deoxyribose nucleic acid are insufficient for a rigorous test of our
structure. So far as we can tell, it is roughly compatible with the experimental
data, but it must be regarded as unproved until it has been checked against
more exact results.
Some of these are given in
the following communications (10)
. We were
not
aware of the details of the results presented there when we devised our
structure (11)
, which rests mainly though not entirely on published
experimental data and stereochemical arguments.
It
has not escaped our notice
(12)
that the specific pairing we have postulated immediately suggests a possible
copying mechanism for the genetic material.
Full details of the
structure, including the conditions assumed in building it, together with
a set of coordinates for the atoms,
will be published
elsewhere (13)
.
We are much indebted
to Dr. Jerry Donohue for constant advice and criticism, especially on
interatomic distances. We have also been stimulated by a knowledge of
the general nature of the unpublished experimental results and ideas of
Dr. M. H. F. Wilkins, Dr. R. E. Franklin and their co-workers at King’s
College, London. One of us (J. D. W.) has been aided by a fellowship from
the National Foundation for Infantile Paralysis.
1
Pauling, L., and Corey, R. B.,
Nature,
171, 346 (1953);
Proc.
U.S. Nat. Acad. Sci.,
39, 84 (1953).
2
Furberg, S.,
Acta Chem. Scand.,
6, 634 (1952).
3
Chargaff, E., for references see Zamenhof, S., Brawerman, G., and Chargaff,
E.,
Biochim. et Biophys. Acta,
9, 402 (1952).
4
Wyatt, G. R.,
J.
Gen. Physiol.
, 36, 201 (1952).
5
Astbury, W. T., Symp. Soc. Exp. Biol. 1, Nucleic Acid, 66 (Camb. Univ.
Press, 1947).
6
Wilkins, M. H. F., and Randall, J. T.,
Biochim. et Biophys. Acta,
10, 192 (1953).
Annotations
(1)
It’s no surprise that James D. Watson and Francis H. C.
Crick spoke of finding the structure of DNA within minutes of their first
meeting at the Cavendish Laboratory in Cambridge, England, in 1951. Watson,
a 23-year-old geneticist, and Crick, a 35-year-old former physicist studying
protein structure for his doctorate in biophysics, both saw DNA’s
architecture as the biggest question in biology. Knowing the structure
of this molecule would be the key to understanding how genetic information
is copied. In turn, this would lead to finding cures for human diseases.
Aware of these
profound implications, Watson and Crick were obsessed with the problem—and,
perhaps more than any other scientists, they were determined to find the
answer first. Their competitive spirit drove them to work quickly, and
it undoubtedly helped them succeed in their quest.
Watson and
Crick’s rapport led them to speedy insights as well. They incessantly
discussed the problem, bouncing ideas off one another. This was especially
helpful because each one was inspired by different evidence. When the
visually sensitive Watson, for example, saw a cross-shaped pattern of
spots in an X-ray photograph of DNA, he knew DNA had to be a double helix.
From data on the symmetry of DNA crystals, Crick, an expert in crystal
structure, saw that DNA’s two chains run in opposite directions.
Since the groundbreaking
double helix discovery in 1953, Watson has used the same fast, competitive
approach to propel a revolution in molecular biology. As a professor at
Harvard in the 1950s and 1960s, and as past director and current president
of Cold Spring Harbor Laboratory, he tirelessly built intellectual arenas—groups
of scientists and laboratories—to apply the knowledge gained from
the double helix discovery to protein synthesis, the genetic code, and
other fields of biological research. By relentlessly pushing these fields
forward, he also advanced the view among biologists that solving major
health problems requires research at the most fundamental level of life.
(2)
On this date,
Nature
published the paper you are reading.
According to
science historian Victor McElheny of the Massachusetts Institute of Technology,
this date was a turning point in a longstanding struggle between two camps
of biology, vitalism and reductionism. While vitalists studied whole organisms
and viewed genetics as too complex to understand fully, reductionists
saw deciphering fundamental life processes as entirely possible—and
critical to curing human diseases. The discovery of DNA’s double-helix
structure was a major blow to the vitalist approach and gave momentum
to the reductionist field of molecular biology.
Historians
wonder how the timing of the DNA race affected its outcome. Science, after
years of being diverted to the war effort, was able to focus more on problems
such as those affecting human health. Yet, in the United States, it was
threatened by a curb on the free exchange of ideas. Some think that American
researcher Linus Pauling would have beaten Watson and Crick to the punch
if Pauling’s ability to travel had not been hampered in 1952 by
the overzealous House Un-American Activities Committee.
(3)
Nature
(founded in 1869)——and hundreds of other scientific journals—help
push science forward by providing a venue for researchers to publish and
debate findings. Today, journals also validate the quality of this research
through a rigorous evaluation called peer review. Generally at least two
scientists, selected by the journal’s editors, judge the quality
and originality of each paper, recommending whether or not it should be
published.
Science publishing
was a different game when Watson and Crick submitted this paper to
Nature.
With no formal review process at most journals, editors usually reached
their own decisions on submissions, seeking advice informally only when
they were unfamiliar with a subject.
(4) The effort to discover the structure of DNA was a race among several players. They were world-renowned chemist Linus Pauling at the California Institute of Technology, and X-ray crystallographers Maurice Wilkins and Rosalind Franklin at King’s College London, in addition to Watson and Crick at the Cavendish Laboratory, Cambridge University.
The competitive juices
were flowing well before the DNA sprint was in full gear. In 1951, Pauling
narrowly beat scientists at the Cavendish Lab, a top center for probing
protein structure, to the discovery that certain proteins are helical.
The defeat stung. When Pauling sent a paper to be published in early 1953
that proposed a three-stranded DNA structure, the head of the Cavendish
gave Watson and Crick permission to work full-time on DNA’s structure.
Cavendish was not about to lose twice to Pauling.
Pauling's proposed structure of DNA was a three-stranded helix with the
bases facing out. While the model was wrong, Watson and Crick were sure
Pauling would soon learn his error, and they estimated that he was six
weeks away from the right answer. Electrified by the urgency—and
by the prospect of beating a science superstar—Watson and Crick discovered
the double helix after a four-week frenzy of model building.
Pauling was
foiled in his attempts to see X-ray photos of DNA from King's College—crucial
evidence that inspired Watson's vision of the double helix—and had
to settle for inferior older photographs. In 1952, Wilkins and the head
of the King's laboratory had denied Pauling's request to view their photos.
Pauling was planning to attend a science meeting in London, where he most
likely would have renewed his request in person, but the United States
House Un-American Activities Committee halted Pauling’s trip, citing
his antiwar activism. It was fitting, then, that Pauling, who won the
Nobel Prize in Chemistry in 1954, also won the Nobel Peace Prize in 1962,
the same year Watson and Crick won their Nobel Prize for discovering the
double helix.
(5) Here, the young scientists Watson and Crick call their model “radically different” to strongly set it apart from the model proposed by science powerhouse Linus Pauling. This claim was justified. While Pauling’s model was a triple helix with the bases sticking out, the Watson-Crick model was a double helix with the bases pointing in and forming pairs of adenine (A) with thymine (T), and cytosine (C) with guanine (G).
(6)
This central
description of the double helix model still stands today—a monumental
feat considering that the vast majority of research findings are either
rejected or changed over time.
According to
science historian Victor McElheny of the Massachusetts Institute of Technology,
the staying power of the double helix theory puts it in a class with Newton’s
laws of motion. Just as Newtonian physics has survived centuries of scientific
scrutiny to become the foundation for today’s space programs, the
double helix model has provided the bedrock for several research fields
since 1953, including the biochemistry of DNA replication, the cracking
of the genetic code, genetic engineering, and the sequencing of the human
genome.
(7) Norwegian scientist Sven Furberg’s DNA model—which correctly put the bases on the inside of a helix—was one of many ideas about DNA that helped Watson and Crick to infer the molecule’s structure. To some extent, they were synthesizers of these ideas. Doing little laboratory work, they gathered clues and advice from other experts to find the answer. Watson and Crick’s extraordinary scientific preparation, passion, and collaboration made them uniquely capable of this synthesis.
(8) A visual representation of Watson and Crick’s model was crucial to show how the components of DNA fit together in a double helix. In 1953, Crick’s wife, Odile, drew the diagram used to represent DNA in this paper. Scientists use many different kinds of visual representations of DNA.
(9) The last hurdle for Watson and Crick was to figure out how DNA’s four bases paired without distorting the helix. To visualize the answer, Watson built cardboard cutouts of the bases. Early one morning, as Watson moved the cutouts around on a tabletop, he found that only one combination of base molecules made a DNA structure without bulges or strains. As Crick put it in his book What Mad Pursuit, Watson solved the puzzle “not by logic but serendipity.” Watson and Crick picked up this model-building approach from eminent chemist Linus Pauling, who had successfully used it to discover that some proteins have a helical structure.
(10) Alongside the Watson-Crick paper in the April 25, 1953, issue of Nature were separately published papers by scientists Maurice Wilkins and Rosalind Franklin of King’s College, who worked independently of each other. The Wilkins and Franklin papers described the X-ray crystallography evidence that helped Watson and Crick devise their structure. The authors of the three papers, their lab chiefs, and the editors of Nature agreed that all three would be published in the same issue.
The “following communications” that our authors are referring to are the papers by Franklin and Wilkins, published on the journal pages immediately after Watson and Crick’s paper. They (and other papers) can be downloaded as PDF files from Nature’s 50 Years of DNA website.
Here are the direct
links:
Molecular
Configuration in Sodium Thymonucleate
Franklin, R., and Gosling, R. G.
Nature
171, 740-741 (1953)
Molecular
Structure of Deoxypentose Nucleic Acids
Wilkins, M. H. F., Stokes, A. R., & Wilson, H. R.
Nature
171, 738-740 (1953)
(11)
This
sentence marks what many consider to be an inexcusable failure to give
proper credit to Rosalind Franklin, a King’s College scientist.
Watson and Crick are saying here that they “were not aware of”
Franklin’s unpublished data, yet Watson later admits in his book
The Double Helix
that these data were critical in solving the
problem. Watson and Crick knew these data would be published in the same
April 25 issue of
Nature,
but they did not formally acknowledge
her in their paper.
What exactly were these data, and how did Watson and Crick gain access
to them? While they were busy building their models, Franklin was at work
on the DNA puzzle using X-ray crystallography, which involved taking X-ray
photographs of DNA samples to infer their structure. By late February
1953, her analysis of these photos brought her close to the correct DNA
model.
But Franklin was frustrated with an inhospitable environment at King’s, one that pitted her against her colleagues. And in an institution that barred women from the dining room and other social venues, she was denied access to the informal discourse that is essential to any scientist’s work. Seeing no chance for a tolerable professional life at King’s, Franklin decided to take another job. As she was preparing to leave, she turned her X-ray photographs over to her colleague Maurice Wilkins (a longtime friend of Crick).
Then, in perhaps the most pivotal moment in the search for DNA’s structure, Wilkins showed Watson one of Franklin’s photographs without Franklin’s permission. As Watson recalled, “The instant I saw the picture my mouth fell open and my pulse began to race.” To Watson, the cross-shaped pattern of spots in the photo meant that DNA had to be a double helix.
Was it unethical for Wilkins to reveal the photographs? Should Watson and Crick have recognized Franklin for her contribution to this paper? Why didn’t they? Would Watson and Crick have been able to make their discovery without Franklin’s data? For decades, scientists and historians have wrestled over these issues.
To read more about
Rosalind Franklin and her history with Wilkins, Watson, and Crick, see
the following:
“Light on a Dark Lady” by Anne Piper, a lifelong friend of
Franklin’s
“The Double Helix and the Wronged Heroine,” an essay on
Nature’s
“Double Helix: 50 years of DNA” Web site
A review of Brenda Maddox’s recent book,
Rosalind Franklin:
The Dark Lady of DNA
in
The Guardian
(UK)
URL:
http://books.guardian.co.uk/whitbread2002/story/0,12605,842764,00.html
(12) This phrase and the sentence it begins may be one of the biggest understatements in biology. Watson and Crick realized at the time that their work had important scientific implications beyond a “pretty structure.” In this statement, the authors are saying that the base pairing in DNA (adenine links to thymine and guanine to cytosine) provides the mechanism by which genetic information carried in the double helix can be precisely copied. Knowledge of this copying mechanism started a scientific revolution that would lead to, among other advances in molecular biology, the ability to manipulate DNA for genetic engineering and medical research, and to decode the human genome, along with those of the mouse, yeast, fruit fly, and other research organisms.
(13) This paper is short because it was intended only to announce Watson and Crick’s discovery, and because they were in a competitive situation. In January 1954, they published the “full details” of their work in a longer paper (in Proceedings of the Royal Society). This “expound later” approach was usual in science in the 1950s as it continues to be. In fact, Rosalind Franklin did the same thing, supplementing her short April 25 paper with two longer articles.
Today, scientists publish their results in a variety of formats. They also present their work at conferences. Watson reported his and Crick’s results at the prestigious annual symposium at Cold Spring Harbor Laboratory in June 1953. As part of our recognition of the fiftieth anniversary of the double helix discovery, we will join scientists at Cold Spring Harbor as they present their papers at the “Biology of DNA” conference .
© exploratorium