H ave you ever tried to blow a bubble with pure water? It won't work. There is a common misconception that water does not have the necessary surface tension to maintain a bubble and that soap increases it, but in fact soap decreases the pull of surface tension - typically to about a third that of plain water. The surface tension in plain water is just too strong for bubbles to last for any length of time. One other problem with pure water bubbles is evaporation: the surface quickly becomes thin, causing them to pop.
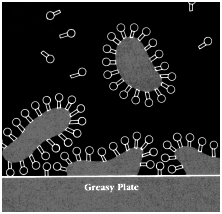
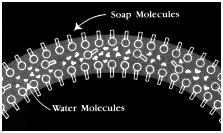
I n a soap-and-water solution the hydrophobic (greasy) ends of the soap molecule do not want to be in the liquid at all. Those that find their way to the surface squeeze their way between the surface water molecules, pushing their hydrophobic ends out of the water. This separates the water molecules from each other. Since the surface tension forces become smaller as the distance between water molecules increases, the intervening soap molecules decrease the surface tension. If that over-filled cup of water mentioned earlier were lightly touched with a slightly soapy finger, the pile of water would immediately spill over the edge of the cup; the surface tension "skin" is no longer able to support the weight of the water because the soap molecules separated the water molecules, decreasing the attractive force between them.
Pop up to bubbles contents |