I f you could see molecules of water and how they act, you would notice that each water molecule electrically attracts its neighbors. Each has two hydrogen atoms and one oxygen atom, H 2 0. The extraordinary stickiness of water is due to the two hydrogen atoms, which are arranged on one side of the molecule and are attracted to the oxygen atoms of other nearby water molecules in a state known as "hydrogen bonding." (If the molecules of a liquid did not attract one another, then the constant thermal agitation of the molecules would cause the liquid to instantly boil or evaporate.
W
ithin the water, at least a few molecules away from the surface, every molecule is engaged in a tug of war with its neighbors on every side. For every "up" pull there is a "down" pull, and for every "left" pull there is a "right" pull, and so on, so that any given molecule feels no net force at all. At the surface things are different. There is no up pull for every down pull, since of course there is no liquid above the surface; thus the surface molecules tend to be pulled back into the liquid. It takes work to pull a molecule up to the surface. If the surface is stretched - as when you blow up a bubble - it becomes larger in area, and more molecules are dragged from within the liquid to become part of this increased area. This "stretchy skin" effect is called surface tension.
Surface tension plays an important role in the way liquids behave. If you fill a glass with water, you will be able to add water above the rim of the glass because of surface tension.
Pop up to bubbles contents |
 H
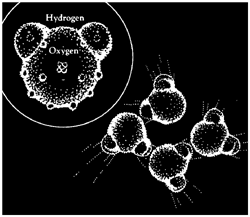
ydrogen atoms have single electrons which tend to spend a lot of their time "inside" the water molecule, toward the oxygen atom, leaving their outsides naked, or positively charged. The oxygen atom has eight electrons, and often a majority of them are around on the side away from the hydrogen atoms, making this face of the atom negatively charged. Since opposite charges attract, it is no surprise that the hydrogen atoms of one water molecule like to point toward the oxygen atoms of other molecules. Of course in the liquid state, the molecules have too much energy to become locked into a fixed pattern; nevertheless, the numerous temporary "hydrogen bonds" between molecules make water an extraordinarly sticky fluid.
H
ydrogen atoms have single electrons which tend to spend a lot of their time "inside" the water molecule, toward the oxygen atom, leaving their outsides naked, or positively charged. The oxygen atom has eight electrons, and often a majority of them are around on the side away from the hydrogen atoms, making this face of the atom negatively charged. Since opposite charges attract, it is no surprise that the hydrogen atoms of one water molecule like to point toward the oxygen atoms of other molecules. Of course in the liquid state, the molecules have too much energy to become locked into a fixed pattern; nevertheless, the numerous temporary "hydrogen bonds" between molecules make water an extraordinarly sticky fluid.