Bouncing Balls
When baseball was in its infancy (before 1848), the ball had plenty of "bounce." The earliest baseballs had a rubber core and were somewhat smaller. Today's baseball is 9 to 9 1/4 inches in circumference, is made up of layers of yarn over a rubber-coated cork center and may not seem to have bounce to it. If you drop a ball on the field it won't bounce back -- a line drive or a strong throw will get more of a bounce out of a baseball. This activity will give you an idea of just how bouncy a baseball is.
What You Need
- Two baseballs and/or golf balls
- A freezer
Directions
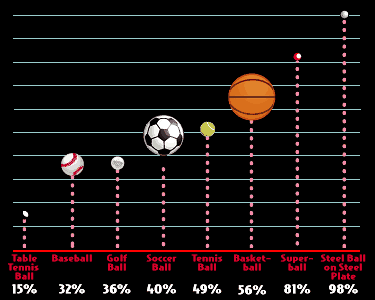
Baseballs have less bounce than tennis balls or golf balls. This is due, in large part, to their construction. To measure the bounciness of a ball, you can try dropping it from a height onto a hard surface. Try comparing a baseball to a golf ball or a tennis ball.
You can also change how a ball bounces by changing its temperature. If you have two baseballs, try putting one in the freezer for an hour and leaving the other at room temperature. Try comparing their bouciness again.
You should notice that the room temperature ball bounces slightly higher. The cold ball should bounce about 80 percent as high. While this is not that dramatic, it's enough to see that temperature can be a factor. Think about those catches players make near the fence; temperature could make the difference between a home run and a long out.
For slightly better results, try this same experiment with golf balls. The refrigerated ball should bounce about 70 percent as high. Or try this experiment with hockey pucks. In the game of hockey, the pucks are frozen before every game to reduce their bounciness.
What's Going On?
When you drop a ball, gravity pulls it toward the floor. The ball gains energy of motion, known as kinetic energy . When the ball hits the floor and stops, that energy has to go somewhere. The energy goes into deforming the ball -- from its original round shape to a squashed shape. When the ball deforms, its molecules are stretched apart in some places and squeezed together in others. As they are pushed about, the molecules in the ball collide with and rub across each other.
Exactly what happens to these molecules as they stretch and squeeze depends on what the ball is made of. Suppose you drop a ball of putty. Rather than bouncing, it hits the floor and flattens. All of the organized motion of the falling ball becomes the random motion of jiggling molecules. The random motion of jiggling molecules is a measure of thermal energy . The putty gets warmer, but it doesn't bounce. Putty is inelastic -- it doesn't return to its original shape.
The baseball is more elastic because its core is made from materials that act quite differently than the putty. Most of these materials are made from long-chain polymer molecules . When you hold the ball in your hand, these long molecules are tangled together like a ball of molecular spaghetti. During a collision, these molecules stretch -- but only for a moment. Atomic motions within the rubber molecules then return them toward their original, tangled shape. Much of the energy of the ball's downward motion becomes upward motion as the ball returns to its original shape and bounces into the air.