Cancer: Cells behaving badly




HeLa cells: Immortal cancer in culture
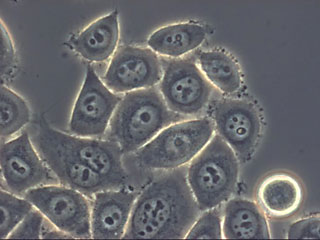
HeLa cells are the workhorses of cancer research. They are derived from cervical cells taken from Henrietta Lacks, a young cancer patient, in 1951. Lacks died of cervical cancer eight months later, but her cells live on in laboratories around the world.
HeLa cells were the first human cells continuously grown in culture. They’ve literally been immortalized: they will continue to grow and divide indefinitely, as long as they’re in the right environment.
Many researchers have used HeLa cells in their labs, particularly in the early days of cancer research, because the cells are readily available and easy to culture. Scientists now have malignant cells from many other tissue types to study.
An important characteristic of HeLa cells—and other cultured cancer cells—is that they are very malleable: they can exist in conditions that would kill other cells, and can adapt to almost any environment. Because they are so robust, HeLa cells have been known to occasionally contaminate other cell lines used for research.
HeLa cells were the first human cells continuously grown in culture. They’ve literally been immortalized: they will continue to grow and divide indefinitely, as long as they’re in the right environment.
Many researchers have used HeLa cells in their labs, particularly in the early days of cancer research, because the cells are readily available and easy to culture. Scientists now have malignant cells from many other tissue types to study.
An important characteristic of HeLa cells—and other cultured cancer cells—is that they are very malleable: they can exist in conditions that would kill other cells, and can adapt to almost any environment. Because they are so robust, HeLa cells have been known to occasionally contaminate other cell lines used for research.
The tiny precancerous lesions that lurk in many of us are a far cry from a dangerous tumor. Most of them will never become cancerous, kept in check by a host of defenses within the body. Even if they do begin to get out of hand, they’ve got a lot of dividing and mutating to do before they become a tumor: most of them would take 10 to 15 years to reach a stage where they could be detected.
At these early, precancerous stages, the number of cells in a lesion is small, and the body has many ways to intercept them. Your immune system, for example, may see precancerous cells as foreign, and kill them. Alternatively, mutations in a young cancer cell may trigger a defense system, directing the cell to commit suicide. Most potentially cancerous cells are, in fact, destroyed. For the ones that slip through, the time needed for them to develop into troublesome tumors provides a window during which these lesions might be taken out of commission by cancer treatments of the future. The key is to identify and attack them. Such early treatments would be a significant improvement over current therapies designed to knock out battle-hardened, surviving cells from an already-problematic tumor, but they will require a better understanding of the earliest changes that provoke cancer.
Many researchers have recently focused their attention on just these moments in a cancer cell’s life. Thea Tlsty, a researcher at the University of California, San Francisco, examines the early events in a cancer cell’s life. By characterizing key changes in a precancerous cell, she hopes to find ways to strike cancers down in their earliest, most vulnerable stages. Tlsty (pronounced TIL-stee) likens a cancerous tumor to a weed. “It has a root and a top to it. The current treatments we use now only take off the top, but you’re left with the root. The cancer regresses, but it can grow back.”
“The new medicine that people are trying to develop would target the root,” she continues. Tlsty studies the role of telomeres , the DNA at the ends of chromosomes, and centrosomes , organelles within the cell that are involved in the separation of chromosomes as the cell divides. For example, while a normally dividing cell has two centrosomes, each pulling one set of chromosomes to each new cell, a cancer cell can have three or four, making the DNA separate unevenly or even making one cell divide into three or more. Variations in the number of centrosomes “...is one of the earliest genetic changes in the development of a cancer cell,” Tlsty explains. “What we really want is for medicine to be predictive and preventative. If we can understand what those first few little changes are and eliminate those cells, the hope is that we can eliminate cancer before it forms.”
At these early, precancerous stages, the number of cells in a lesion is small, and the body has many ways to intercept them. Your immune system, for example, may see precancerous cells as foreign, and kill them. Alternatively, mutations in a young cancer cell may trigger a defense system, directing the cell to commit suicide. Most potentially cancerous cells are, in fact, destroyed. For the ones that slip through, the time needed for them to develop into troublesome tumors provides a window during which these lesions might be taken out of commission by cancer treatments of the future. The key is to identify and attack them. Such early treatments would be a significant improvement over current therapies designed to knock out battle-hardened, surviving cells from an already-problematic tumor, but they will require a better understanding of the earliest changes that provoke cancer.
Many researchers have recently focused their attention on just these moments in a cancer cell’s life. Thea Tlsty, a researcher at the University of California, San Francisco, examines the early events in a cancer cell’s life. By characterizing key changes in a precancerous cell, she hopes to find ways to strike cancers down in their earliest, most vulnerable stages. Tlsty (pronounced TIL-stee) likens a cancerous tumor to a weed. “It has a root and a top to it. The current treatments we use now only take off the top, but you’re left with the root. The cancer regresses, but it can grow back.”
“The new medicine that people are trying to develop would target the root,” she continues. Tlsty studies the role of telomeres , the DNA at the ends of chromosomes, and centrosomes , organelles within the cell that are involved in the separation of chromosomes as the cell divides. For example, while a normally dividing cell has two centrosomes, each pulling one set of chromosomes to each new cell, a cancer cell can have three or four, making the DNA separate unevenly or even making one cell divide into three or more. Variations in the number of centrosomes “...is one of the earliest genetic changes in the development of a cancer cell,” Tlsty explains. “What we really want is for medicine to be predictive and preventative. If we can understand what those first few little changes are and eliminate those cells, the hope is that we can eliminate cancer before it forms.”