|
What
is sugar?
 The
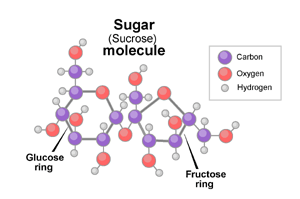
white stuff we know as sugar is sucrose, a molecule composed
of 12 atoms of carbon, 22 atoms of hydrogen, and 11 atoms
of oxygen (C
12
H
22
O
11
).
Like all compounds made from these three elements, sugar
is a carbohydrate. It’s found naturally in most plants,
but especially in sugarcane and sugar beets—hence their
names.
The
white stuff we know as sugar is sucrose, a molecule composed
of 12 atoms of carbon, 22 atoms of hydrogen, and 11 atoms
of oxygen (C
12
H
22
O
11
).
Like all compounds made from these three elements, sugar
is a carbohydrate. It’s found naturally in most plants,
but especially in sugarcane and sugar beets—hence their
names.
Sucrose
is actually two simpler sugars stuck together: fructose
and glucose. In recipes, a little bit of acid (for example,
some lemon juice or cream of tartar) will cause sucrose
to break down into these two components.
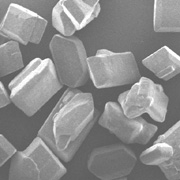
If
you look closely at dry sugar, you’ll notice it comes
in little cubelike shapes. These are sugar crystals, orderly
arrangements of sucrose molecules.
|
Under
a microscope, you can see that sugar crystals aren’t
cubes, exactly, but oblong and slanted at both ends.
(Image
courtesy of Nutrition and Food Management Dept., Oregon
State University)
|
What
happens when you heat a sugar solution?
When
you add sugar to water, the sugar crystals dissolve and
the sugar goes into solution. But you can’t dissolve
an infinite amount of sugar into a fixed volume of water.
When as much sugar has been dissolved into a solution as
possible, the solution is said to be saturated.
The
saturation point is different at different temperatures.
The higher the temperature, the more sugar that can be held
in solution.
When
you cook up a batch of candy, you cook sugar, water, and
various other ingredients to extremely high temperatures.
At these high temperatures, the sugar remains in solution,
even though much of the water has boiled away. But when
the candy is through cooking and begins to cool, there is
more sugar in solution than is normally possible. The solution
is said to be supersaturated with sugar.
Supersaturation
is an unstable state. The sugar molecules will begin to
crystallize back into a solid at the least provocation.
Stirring or jostling of any kind can cause the sugar to
begin crystallizing.
Why
are crystals undesirable in some candy recipes—and
how do you stop them from forming?

|
|
Interfering
agents
(Image courtesy of Nutrition and Food Management Dept.,
Oregon State University)
|
The
fact that sugar solidifies into crystals is extremely important
in candy making. There are basically two categories of candies
-
crystalline
(candies which contain crystals in
their finished form, such as fudge and fondant), and
noncrystalline
,
or
amorphous
(candies which do not contain crystals,
such as lollipops, taffy, and caramels). Recipe ingredients
and procedures for noncrystalline candies are specifically
designed to prevent the formation of sugar crystals, because
they give the resulting candy a grainy texture.
One
way to prevent the crystallization of sucrose in candy is
to make sure that there are other types of sugar—usually,
fructose and glucose—to get in the way. Large crystals
of sucrose have a harder time forming when molecules of
fructose and glucose are around. Crystals form something
like Legos locking together, except that instead of Lego
pieces, there are molecules. If some of the molecules are
a different size and shape, they won’t fit together,
and a crystal doesn’t form.
A
simple way to get other types of sugar into the mix is to
"invert" the sucrose (the basic white sugar you
know well) by adding an acid to the recipe. Acids such as
lemon juice or cream of tartar cause sucrose to break up
(or invert) into its two simpler components, fructose and
glucose. Another way is to add a nonsucrose sugar, such
as corn syrup, which is mainly glucose. Some lollipop recipes
use as much as 50% corn syrup; this is to prevent sugar
crystals from ruining the texture.
Fats
in candy serve a similar purpose. Fatty ingredients such
as butter help interfere with crystallization—again,
by getting in the way of the sucrose molecules that are
trying to lock togeter into crystals. Toffee owes its smooth
texture and easy breakability to an absence of sugar crystals,
thanks to a large amount of butter in the mix.
|
